The results of this study were published in the May 2009 issue of the peer review journal, Clinical Drug Investigation.
Hairmax LaserComb® Phototherapy Device in the Treatment of Male Androgenetic Alopecia.
Leavitt M, Charles G, Heyman E, Michaels D
Studies Objectives
The studies were designed to support the 2007 510K submission to the FDA and was subjected to an IRB approval and complied in accordance with GCP (Good Clinical Practices). The objectives of the study in males were to assess the following:
- promotion of hair growth through changes in hair count
- cessation of hair loss
- scalp overall health
- safety
Study Design
This studies was designed as a multi-center, randomized, sham-device controlled trial conducted at multiple sites in the United States. Subjects were to use the device three times per week on non-consecutive days for a total of 26 weeks. Hair count measurements were performed at baseline immediately prior to randomization and again at 8, 16 and 26 weeks.
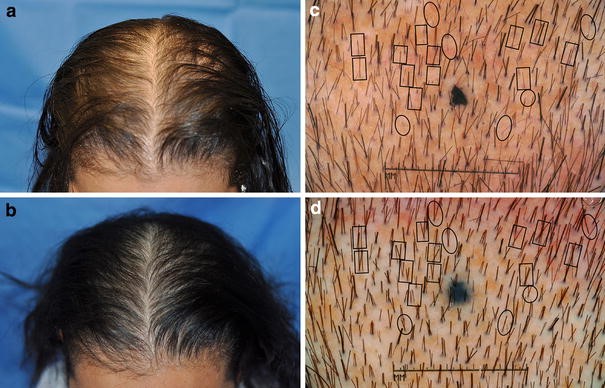
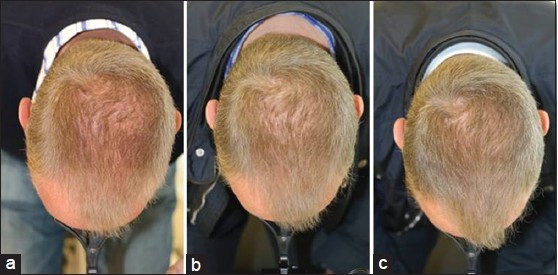
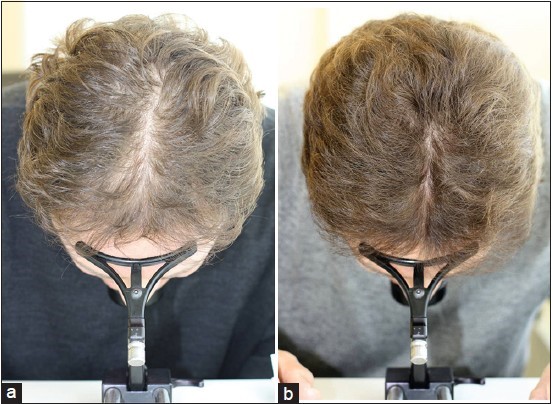
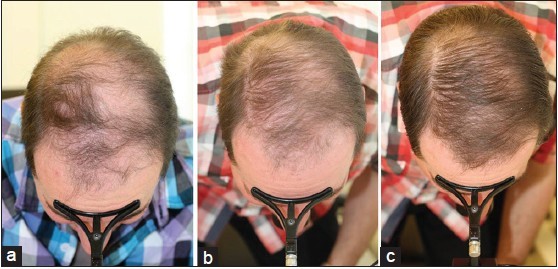
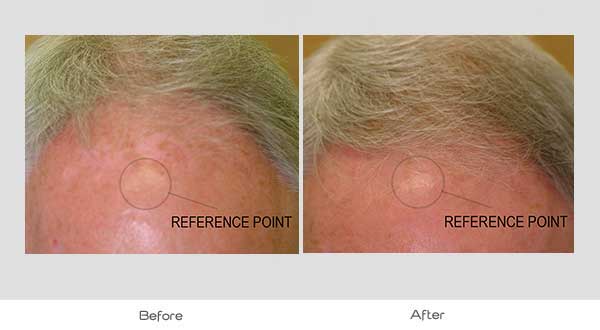
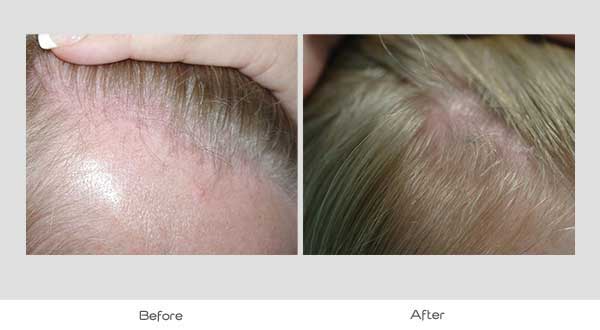
Global Images
Qualified subjects had global images recorded at each visit using a stereotactic device. The global images above correspond with the unretouched Macro images below.
Subject Population and Demographics
The study population was males between the ages of 25 and 60 years with a diagnosis of androgenetic alopecia who had been experiencing active hair loss with the last 12 months. The inclusion criteria required a Norwood-Hamilton classification or IIa to V, and Fitzpatrick Skin Type I to IV. All subjects were randomized. A biostatistician calculated the study to be of a proper size to gauge statistically significant results.
Methods
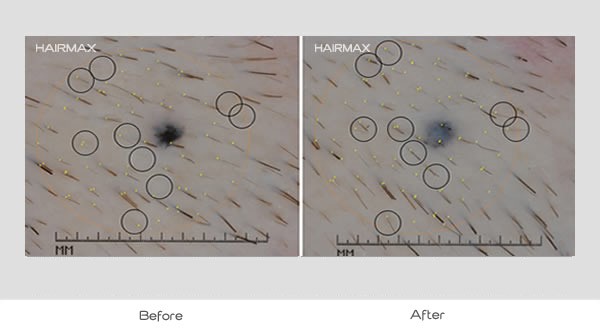
After an assessment of the scalp for androgenetic alopecia and exclusion of other dermatological conditions, subjects were randomized with either active or sham devices. Subjects were then photographed for global evaluation, had the target site of the scalp identified and tattooed for baseline density and were given the device without investigation usage instructions per protocol for OTC use. Subjects returned to the clinic at 8 and 16 weeks with a final visit at week 26 for clinical evaluation.
RESULTS
110 Subjects completed study:
- All subjects exhibited a significantly greater increase in mean terminal hair density than subjects in sham device group (p<0.0001)
- Patients subjective assessment showed significant improvement in hair regrowth
- No serious adverse events were reported
GLOBAL IMAGES – Before and After